Vero Cell Sinopharm Side Effects, Coronavirus Confusion Over Efficacy Of Chinese Vaccines Science In Depth Reporting On Science And Technology Dw 13 04 2021
Its easy storage requirements make it highly suitable for low-resource settings. What are the side effects of Sinopharm vaccine.
Worries Around Sinopharm Resurface Following Low Antibody Tests
The real name of the vaccine is BBIBP-CorV.
Vero cell sinopharm side effects. My left arm where I got the shot is sore. Vietnam approved emergency use of the vaccine on june 4. Based on clinical trials vaccine safety can be tolerated with a frequency of occurrence of each side effect 001 percent or classified as very rare.
The mutant novel coronavirus strains cases were found in Shanghai Guangdong and. Headaches fatigue injection site reactions. May affect 01-1 people.
These normal vaccine reactions are usually mild and subside after a few days. I do feel okay now. Why nobody wants Vero cell in Vietnam.
There was no effect on the fertility of parental female and male rats no toxicity and teratogenicity of embryo fetus and no effect on the growth and development of F1 generation pups. But as with other inactivated vaccines the side effects appear to be mild. 2Vero Cell o Sinopharm.
If anything the vaccine choice has added to. Fatigue fever headache and aching limbs are also not uncommon in the first three days after vaccination. Sinopharm vaccine side effects There are no published studies yet that have focused on Sinopharm vaccine side effects.
These might include swelling pain redness an itchy rash and other mild forms of irritation. Vero cell is the vaccination produced by Sinopharm. This happens because it is the most visible phrase that is not written in Chinese on the packaging.
Whereas in subjects over 60 years of age there were no reports of grade 3 local side effects. I went home and napped for two hours. The Sinopharm product is an inactivated vaccine called SARS-CoV-2 Vaccine Vero Cell.
Common effects include fever fatigue cough nausea diarrhea or itching. Sinopharms SARS-CoV-2 strain WIV04 strain and GenBank number MN996528 was isolated from a patient in the Jinyintan Hospital Wuhan China. La llamada vacuna vero cell es de sinopharm.
What can be the side effects. There may also be redness at the injection site edema or swelling. I will see how I feel tomorrow but from what I read online Vero cell has the least side effects since it is an inactivated vaccine.
Its overall efficacy rate is 86 percent. Based on these data the most common side effects were. The side effects associated with vaccination in volunteers include pain at the injection site headache high temperature or fatigue.
Among the most frequent and temporary side effects are pain at the injection site and headache. May affect 1-10 people injection site swelling pruritus erythema induration myalgia nausea diarrhea arthralgia cough chills pruritus loss of appetite rhinorrhea sore throat nasal congestion abdominal pain. A person might also experience side effects around the injection site which is usually the upper arm.
The virus was cultivated in a qualified Vero cell line for propagation and the supernatant of the infected cells was inactivated with β-propiolactone 14000 volvol at 2 to 8 C for 48 hours. Anecdotally people report a sore arm and minor swelling with others feeling fatigue or symptoms similar to a cold which pass in a few days. Sinopharm CNBG Covid-19 Vaccine is a broad-spectrum protection and is stepping up its testing on mutant strains Q.
COVID-19 Vaccine Vero Cell Inactivated. Sinovac spokesman Liu Peicheng told Reuters preliminary results based on blood samples from those vaccinated with its shot showed a three-fold reduction in neutralizing effect against the Delta. My stamped form.
Prevalence of the general side effects after 2 nd dose Sinopharm COVID-19 vaccination among 2 group of participants 49 years old vs 49 years old no. Some of these side effects were slightly more frequent in adolescents 12 to 15 years than in adults. In Venezuela the use of the name has spread Vero Cell to refer to this vaccine developed by Sinopharm.
Based on the available data the Sinopharm vaccine is reported to have 100 percent protection from moderate and severe forms of Covid-19. It is also found as COVID-19 BIBP vaccine or simply Sinopharm. The interim recommendations and the background document are also available online.
Common may affect up to 1 in 10 people Injection Site Swelling Pruritus Erythema Induration.
Who Experts Voice Very Low Confidence In Some Sinopharm Covid 19 Vaccine Data Reuters

Questions And Answers On Sinopharm Covid 19 Vaccine Bangkok Hospital

China S Sinopharm Vaccine Most Unsafe In World With 73 Side Effects Taiwan News 2021 01 06 18 38 00

Who Approves A Covid 19 Vaccine From China S Sinopharm For Emergency Use

China S Covid Vaccine From Sinopharm Is 86 Effective Uae Says

Sinopharm Bibp Covid 19 Vaccine Wikipedia

Full List Of Adverse Reactions From China S Sinopharm Vaccine Revealed Taiwan News 2021 01 11 09 17 00
Uae Says Sinopharm Vaccine Has 86 Efficacy Against Covid 19 Reuters
Voa Twisted Words On Sinopharm Vaccine To Support Own Narrative To Discredit Chinese Products Expert Global Times

Coronavirus Confusion Over Efficacy Of Chinese Vaccines Science In Depth Reporting On Science And Technology Dw 13 04 2021
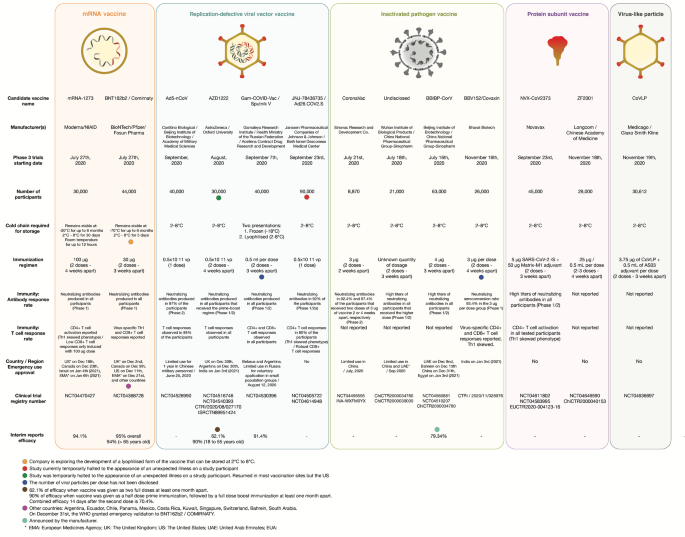
Sars Cov 2 Vaccines Strategies A Comprehensive Review Of Phase 3 Candidates Npj Vaccines

Vaccine Diplomacy Sees Egypt Roll Out Chinese Coronavirus Jab Global Health The Guardian

Hungary Rolls Out China S Sinopharm Jab Amid Lagging Trust

Who Approval Of Chinese Vaccine Will Largely Accelerate Covax Supply Analysts Global Times

Sinopharm Vero Cell Inactivated Covid 19 Vaccine
Sinopharm Vaccine Side Effects Price Efficacy Of China S Sinopharm Covid Vaccine India News Times Of India

Coronavirus How Effective Are The Chinese Vaccines Science In Depth Reporting On Science And Technology Dw 01 02 2021
Egypt Reports No Serious Side Effects On Chinese Covid 19 Vaccine Cgtn

Safety Tolerability And Immunogenicity Of An Inactivated Sars Cov 2 Vaccine In Healthy Adults Aged 18 59 Years A Randomised Double Blind Placebo Controlled Phase 1 2 Clinical Trial The Lancet Infectious Diseases
