Vero Cell Beijing And Sinopharm Vaccine Are Same, Sinopharm Vero Cell Inactivated Covid 19 Vaccine
COVID-19 Vaccine Vero Cell Inactivated Sinopharm CO1 accne Explaner 4 MAY 1 Recommended for age 18 years of age and above Vaccination of children or adolescents below the age of 18 years is not routinely recommended although the studies are underway. The vaccine called Vero is based on an inactivated virus.
China S Sinopharm Reports Strong Interim Results For Its Covid 19 Vaccine South China Morning Post
The inactivated SARS-CoV-2 Vaccine Vero cell manufactured by WIBP Experimental.

Vero cell beijing and sinopharm vaccine are same. Instramuscular upper arm 18 to 60 years Name SARS-CoV-2 Vaccine Vero Cell Inactivated produced by Beijing Institu te of Biological products. Its easy storage requirements make it highly suitable for low-resource settings. Disodium hydrogen phosphate dodecahydra te sodium dihydrogen phosphate monohydrate sodium chloride DESCRIPTION CoronaVac is a milky-white suspension.
First dose of the vaccine means that person cannot take the vaccine Administration. Investigational Vaccine 2 Participants will receive 2 doses of the inactivated SARS-CoV-2 vaccine Vero cell manufactured by BIBP according to the immunization schedule of D0 D21. The company Sinopharm developed it in collaboration with the Wuhan Institute of Virology and the Institute of Biological Products.
One DNA vaccine two RNA vaccines PfizerBioNTech and Moderna nine conventional inactivated vaccines BBIBP-CorV Chinese Academy of Medical Sciences CoronaVac Covaxin CoviVac COVIran Barekat Minhai-Kangtai QazVac and WIBP-CorV five viral vector vaccines Sputnik Light Sputnik V OxfordAstraZeneca Convidecia and Janssen and five protein subunit vaccines. Its product name is SARS-CoV-2 Vaccine Vero Cell not to be confused with the similar product. The WHO guidelines require vaccines to.
Also recommends that anyone who has had an allergic reaction to one dose of a vaccine not receive a second dose of. Sinopharms COVID-19 vaccine. BEIJING June 29 Reuters - Many countries from China to Indonesia and Brazil rely heavily on Chinese vaccines to inoculate their people against COVID-19.
SARS-CoV-2 Vaccine Vero Cells. Peer-reviewed results published in JAMA of Phase III trials in United Arab Emirates and Bahrain showed BBIBP-CorV 781 effective against symptomatic cases and 100 against severe cases 21 cases in vaccinated group vs. 95 cases in placebo group.
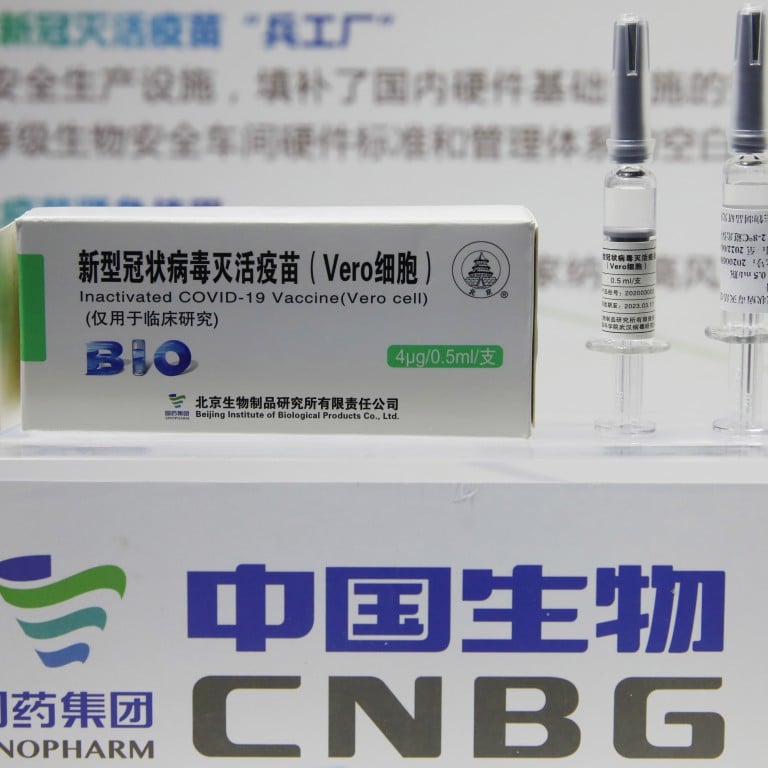
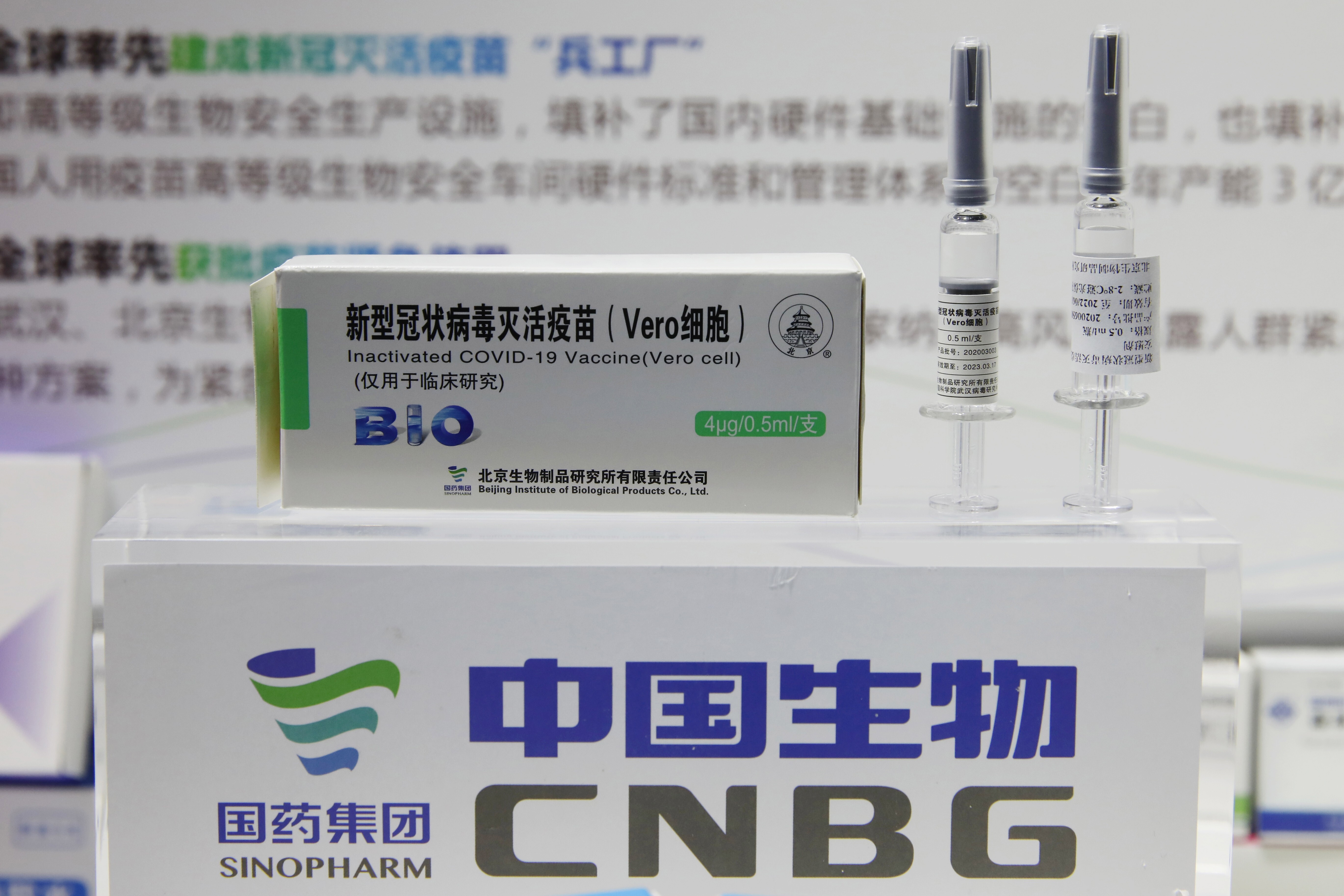
COVID-19 Vaccine Vero Cell Inactivated COMPOSITION Active ingredient. On 9 December 2020 Ministry of Health and Prevention MOHAP announced that it had officially registered the Beijing Institute of Biological Products inactivated vaccine known as Sinopharm vaccine which was developed jointly with Sinopharm - CNBG China National Biotec Group. Twenty vaccines are authorized by at least one national regulatory authority for public use.
Its product name is SARS-CoV-2 Vaccine Vero Cell not to be confused with the similar product name of CoronaVac Tags approve nafdac Sinopharm COVID-19 vaccine. 2 4 Month Usage. The News Agency of Nigeria NAN reports that BBIBP-CorV also known as the Sinopharm COVID-19 vaccine or BIBP vaccine is one of two inactivated virus COVID-19 vaccines.
Recommended schedule 2 doses 05 mL each at a recommended interval of 3 to 4 weeks. Its product name is SARS-CoV-2 Vaccine Vero Cell not to be confused with the similar product name of CoronaVac. It is the also first vaccine that will carry a vaccine vial monitor a small sticker on the vaccine vials that change color as the vaccine is exposed to heat letting health workers know whether the vaccine can be safely used.
Sinopharm CNBGs Covid-19 Vaccine is derived from Vero cell which goes through cultivation inactivation and purification. For the same vaccine. Inactivated SARS-CoV-2 Virus CZ02 s train Adjuvant.
Sinopharm Beijing Inactivated. Product comes in pre-filled syringes or vials. A number of African Latin American and Asian countries have chosen to approve and use the Vero Cell vaccine by the Beijing-based pharmaceutical company Sinovac and the Sinopharm vaccine produced.
The vaccine was developed by Sinopharms Beijing Institute of. Sinopharm has been reported to have a 79 per cent efficacy rate while results from Sinovac have ranged from 504 per cent to 835 per cent. The Sinopharm product is an inactivated vaccine called SARS-CoV-2 Vaccine Vero Cell.
The vaccines immunogenicity safety and immune protection are.

Full List Of Adverse Reactions From China S Sinopharm Vaccine Revealed Taiwan News 2021 01 11 09 17 00

Sinopharm Vero Cell Inactivated Covid 19 Vaccine
/cloudfront-us-east-2.images.arcpublishing.com/reuters/IHA57VIY2RLWNAI4S44IMQPCDI.jpg)
Vietnam Approves China S Sinopharm Vaccine For Use Against Covid 19 Reuters
China S Sinopharm Reports Strong Interim Results For Its Covid 19 Vaccine South China Morning Post

Study Chinese Covid Shot May Offer Elderly Poor Protection
Inside A Beijing Coronavirus Vaccination Center Cnn Video

Questions And Answers On Sinopharm Covid 19 Vaccine Bangkok Hospital

Sinopharm Bibp Covid 19 Vaccine Wikipedia

Who Approves A Covid 19 Vaccine From China S Sinopharm For Emergency Use
China S Sinopharm Vaccine Receives 1st Eu Gmp Certificate Cgtn
Voa Twisted Words On Sinopharm Vaccine To Support Own Narrative To Discredit Chinese Products Expert Global Times
China S Vaccine Diplomacy Assumes Geopolitical Importance Merics

Taiwan Puts All Eggs In One Basket By Refusing Mainland Vaccines Global Times

Vaccine Diplomacy Sees Egypt Roll Out Chinese Coronavirus Jab Global Health The Guardian
Hungary Reaches Deal To Buy China S Sinopharm Vaccine Pm Aide Says Cgtn

Who Approves China S Sinopharm Covid 19 Vaccine For Emergency Use Has 79 Efficacy Coronavirus Outbreak News
Abu Dhabi Launches New Covid 19 Vaccine Plant With China S Sinopharm Cgtn
Who Approves China S Sinopharm Covid Vaccine For Emergency Use
China S Covid Vaccine From Sinopharm Is 86 Effective Uae Says